GI-MAP Inaccurate? We Think Not.
Posted On November 8th, 2022
Response from Diagnostic Solutions Laboratory
We'd like to thank everyone for their interest in the GI-MAP® stool test, especially the thousands of practitioners who trust GI-MAP as an essential tool in their clinical practice.
Diagnostic Solutions Laboratory (DSL) would like to provide some additional education and clarity to some of the points discussed in a recent video that erroneously claimed that the GI-MAP is inaccurate.
- All of the primary criticisms presented in the video can simply be explained by differences in sample density, and also variations in the abundance of individual microbes among different parts of the stool. The fact that most of the markers for each of the "split samples" trended higher or lower in unison clearly indicates that the microbial density of stool in the split samples was not equivalent. (See Figure 1- Sample One Comparison PDF.)
- Stool is a very complex and heterogeneous medium, and producing a true "split" sample is challenging even for controlled laboratory environments. The experiment presented in the video and instructions given to patients (to homogenize by stirring the sample by hand) cannot accurately differentiate between differences in results due to methodology limitations vs. variability in the concentration of stool per sample. We would like to emphasize that all samples sent to the laboratory should be prepared according to the collection instructions included with the kit — this is how the test has been extensively validated.
- The video overlooked the fact that the vast majority of results were consistent between both samples, and the overall clinical picture was essentially the same. The microbial markers included in GI-MAP are assessed with quantitative PCR (qPCR), and each microbial marker has undergone rigorous validation, resulting in 97% consistency across replicates. Intestinal health markers, such as calprotectin, are run using state-of-the-art chemiluminescent platform.
- To clarify, a result of <dl on the GI-MAP does not mean "zero" or that NO microbial DNA was detected. Rather, the lab has set a lower limit of detection (LLOD) for each target individually in addition to the reference range for which the organism is designated "High." This is different from other laboratories and methodologies that only report positive/negative as it relates to predictive value for disease. The samples with higher stool amounts resulted in detection of more low-abundance microbes than samples with lower stools amounts, where the amount of the corresponding markers likely fell below the limit of detection.
- Low-level pathogens or opportunists are often present in asymptomatic individuals. Treatment decisions (to treat or not to treat) should be based on proper patient assessment, clinical judgment, and evaluation of the GI-MAP, including the intestinal health markers.
- In August of 2022, the GI-MAP underwent some enhancements which included the addition of new targets/markers, improved visual reporting, and some reference range adjustments after lab analysis and internal review. Akkermansia muciniphila was one organism for which a reference range change was applied.
- The extensive misrepresentation of GI-MAP test results in this video of "split sampling" was not only obviously biased against Diagnostic Solutions Laboratory, but also potentially unfair to LabCorp. The inflammatory bowel disease (IBD) case presented in the video demonstrates the expected detection of elevated calprotectin by the GI-MAP in an ulcerative colitis symptom flare. The quantitative difference in calprotectin between splits can be accounted for by differences in stool amount per sample.
- Diagnostic Solutions Laboratory offers extensive education and practitioner support, including one-on-one consults for lab interpretation. If the practitioner who offered this misinformation about the GI-MAP had reached out to us for a consultation, they would have been made aware of the obvious differences due to sample amount in each split.
- While we support the efforts of practitioners to better understand the applicability, strengths, and limitations of their clinical laboratory tool kit, we encourage those who wish to publicly question the accuracy of a test to first seek to understand whether their criticisms are in fact accurate and scientifically sound. By contacting DSL customer service directly, practitioners can benefit from our extensive in-house scientific and clinical expertise.
Figure 1- Sample One Comparison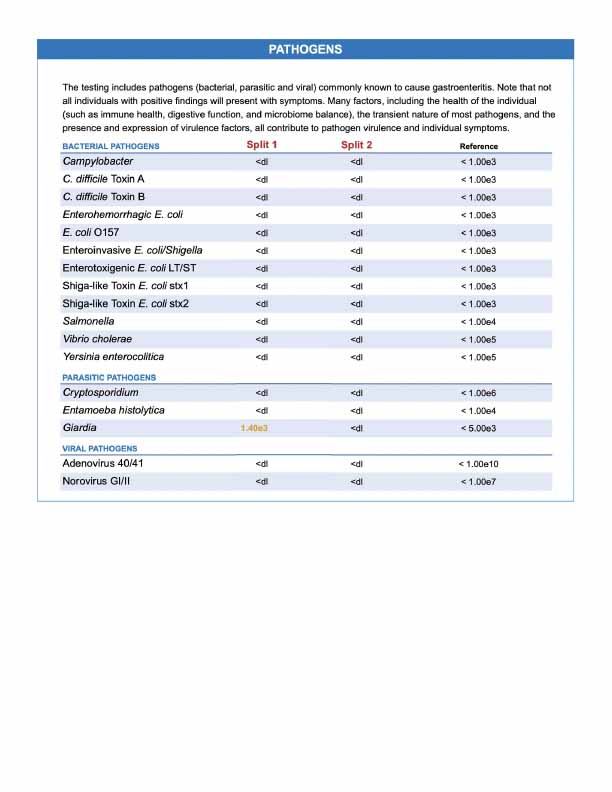
GI-MAP Methodology Guide
Additional Resources
Get in Touch with a Diagnostic Solutions Service Team Member
We're Here to Answer Your Questions
- Contact Us
- Customer Service: 877-485-5336
- Email: cs@diagnosticsolutionslab.com